Carly Eakin, Graduate Researcher, University of Maine
The past three summers I have surveyed wood frog tadpoles and spotted salamander larvae in over 30 vernal pools around Bangor, Maine, collecting data on their population status and individual body conditions. This means handling a lot of tadpoles and larval salamanders: Between me and my steadfast field crew we have carefully measured over 10,000 tadpoles and salamanders. Unfortunately, at several pools the conversation during amphibian body examinations goes something like this:
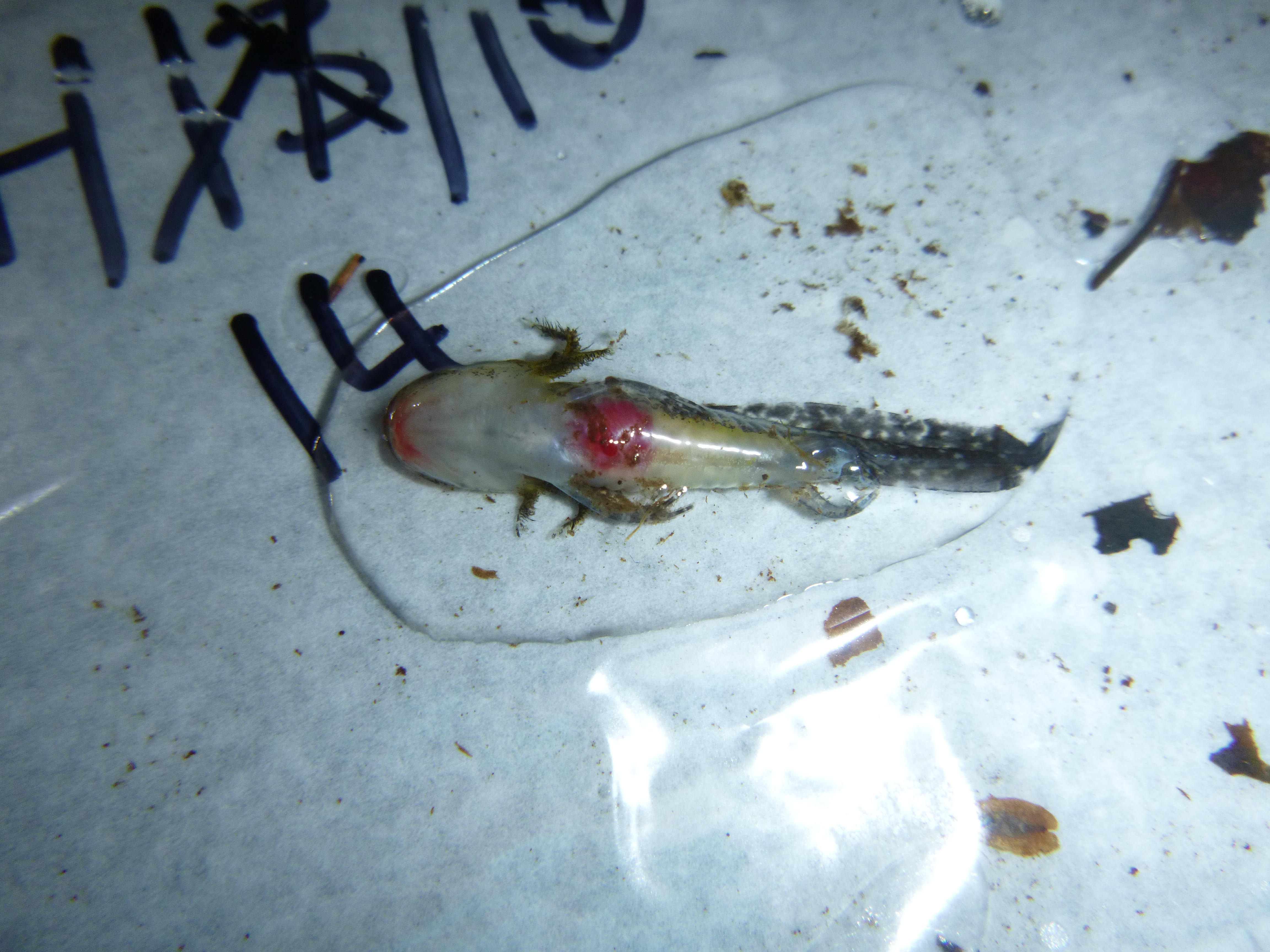
“Uh oh. There’s a bunch of little red spots on this one.”
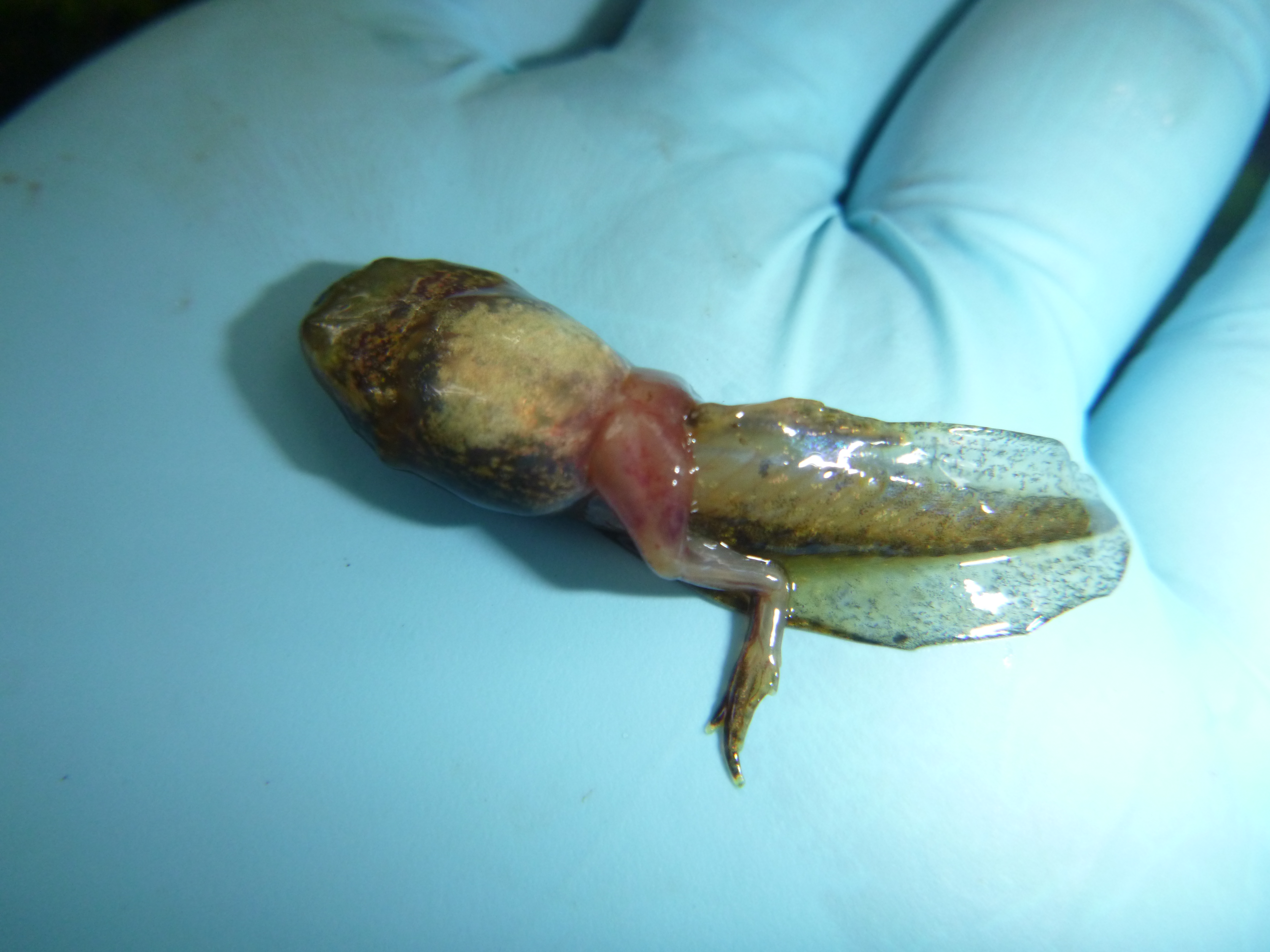
“And this one has huge, swollen red legs.”
“Gross! This tadpole looks like its tail fin is disintegrating!”
“This salamander looks like its heart exploded! Poor guy, he’s already dead.”
I always cringe when this happens because the likely cause of these symptoms is Ranavirus.
These viruses not only affect individual amphibians, but there is substantial evidence that ranaviruses have greatly contributed to population declines for several amphibian species throughout the US and world. Wood frog tadpoles are especially susceptible to ranavirus, with observations of die-offs of entire wood frog tadpole cohorts are not uncommon throughout the Northeastern US. These die-offs can happen suddenly, with a pool of seemingly healthy tadpoles one day and thousands of dead tadpoles two days later. When wood frog tadpoles and salamander larvae become infected they can have one or many externally-visible signs of disease, including hemorrhaging, swollen red legs, skin sloughing/deterioration, bloating, skin ulcers, emaciation, lethargy, and death. These symptoms result from virus-programmed rapid cell death; the virus literally disintegrates internal organs.

Field technician, Diane Dunham, decontaminates waders after an amphibian survey.
Ranavirus is also highly transmissible by direct contact and can persist in pool water or sediments for at least a week. This means that if a tadpole is infected with ranavirus it will likely infect all tadpoles that it ‘bumps’ into, and those tadpoles spread it to all that they bump into, and so on. All this tadpole “bumping” can quickly result all tadpoles in a pool being infected, especially as pools dry and shrink later in the summer and tadpoles become crowded. Because the virus can persist so long in water and sediment, wildlife and humans that travel among multiple pools within a short time may carry virus in water and mud clinging to their fur, feathers, or rubber boots. Knowing that ranavirus was possibly lurking in our study pools, we took precautions against spreading it among pools. After every pool we disinfected all our gear that contacted pool water, brushing out mud stuck in boot treads and thoroughly spraying equipment with bleach or chlorohexidine solution (See here for more information on decontamination procedures).
References
Brunner, JL, KE Barnett, CJ Gosier, SA McNulty, MJ Rubbo, MB Kolozsvary. 2011. Ranavirus infection in die-offs of vernal pool amphibians in New York, USA. Herpetological Review, 42(1), 76–79.
Gahl, MK, & AJK Calhoun. 2010. The role of multiple stressors in ranavirus-caused amphibian mortalities in Acadia National Park wetlands. Canadian Journal of Zoology, 88(1), 108–121.
Haislip, NA, MJ Gray, JT Hoverman, DL Miller. 2011. Development and disease: How susceptibility to an emerging pathogen changes through anuran development. PLoS ONE, 6(7).
Lesbarrères D, A Balseiro, J Brunner, VG Chinchar, A Duffus, J Kerby, DL Miller, J Robert, DM Schock, T Waltzek, MJ Gray. 2012. Ranavirus: past, present and future. Biology Letters, 8, 4:481-483.
USGS National Wildlife Health Center (NWHC). 2015. Ranvirus. http://www.nwhc.usgs.gov/ disease_ information/other_diseases/ranavirus.jsp; accessed 2016 January 13.
Wheelwright NT, MJ Gray, RD Hill, DL Miller. 2014. Sudden mass die-off of a large population of wood frog (Lithobates sylvaticus) tadpoles in Maine, USA, likely due to ranavirus. Herpetological Review, 45:240-242.